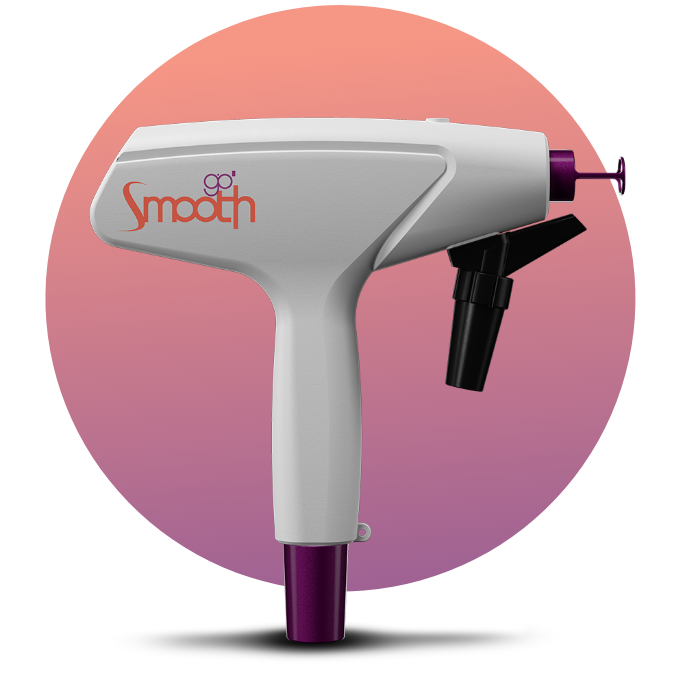

Versatility with 8
LASER and light technologies
The best-selling
Brazilian LASER
platform in the world.
EXCLUSIVE HANDPIECES
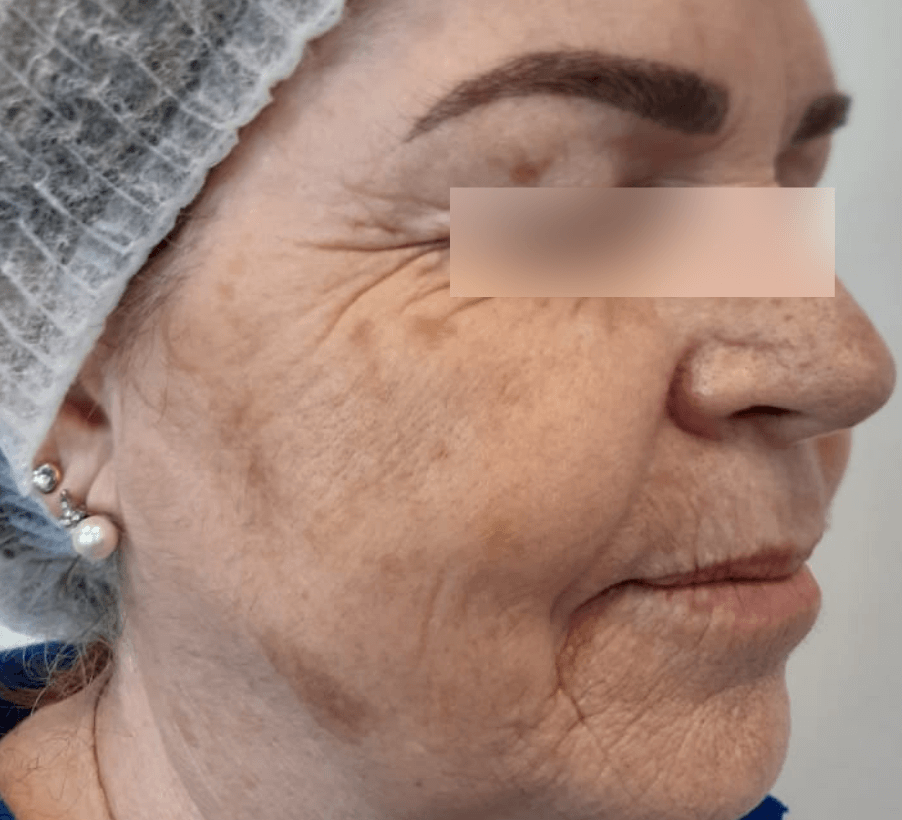
SOME RESULTS
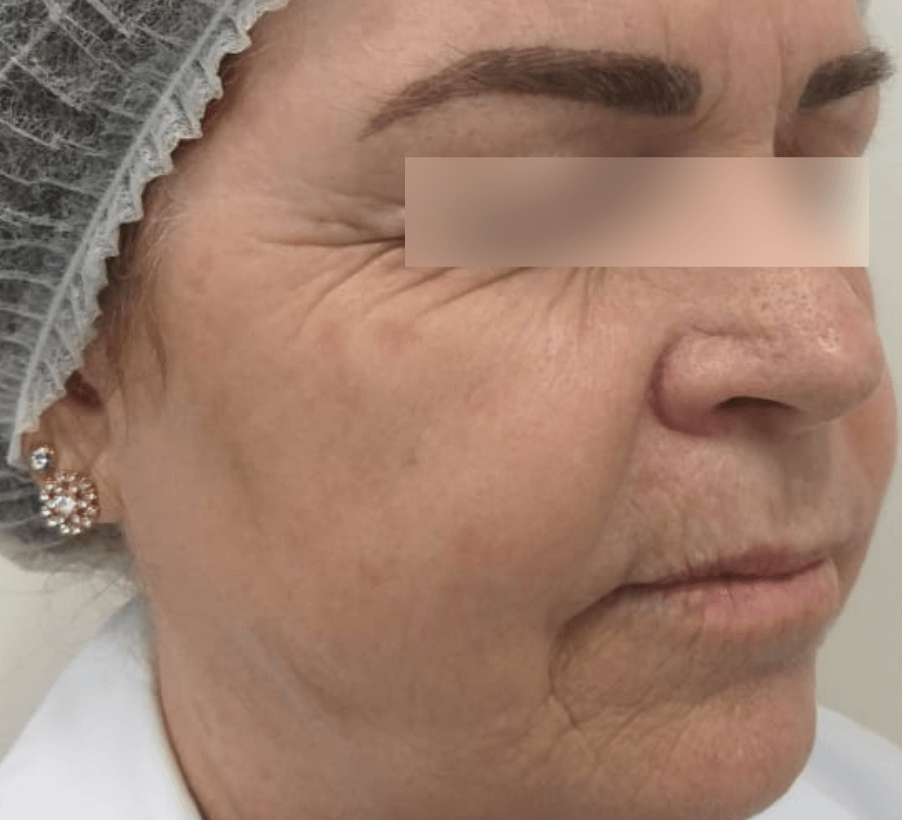
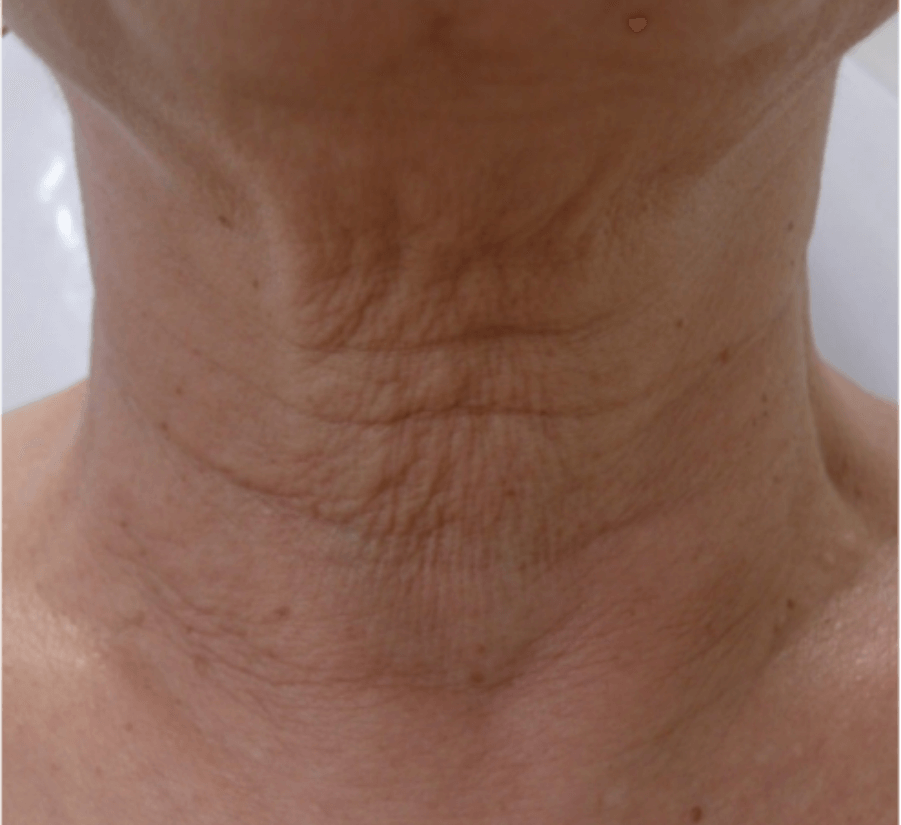
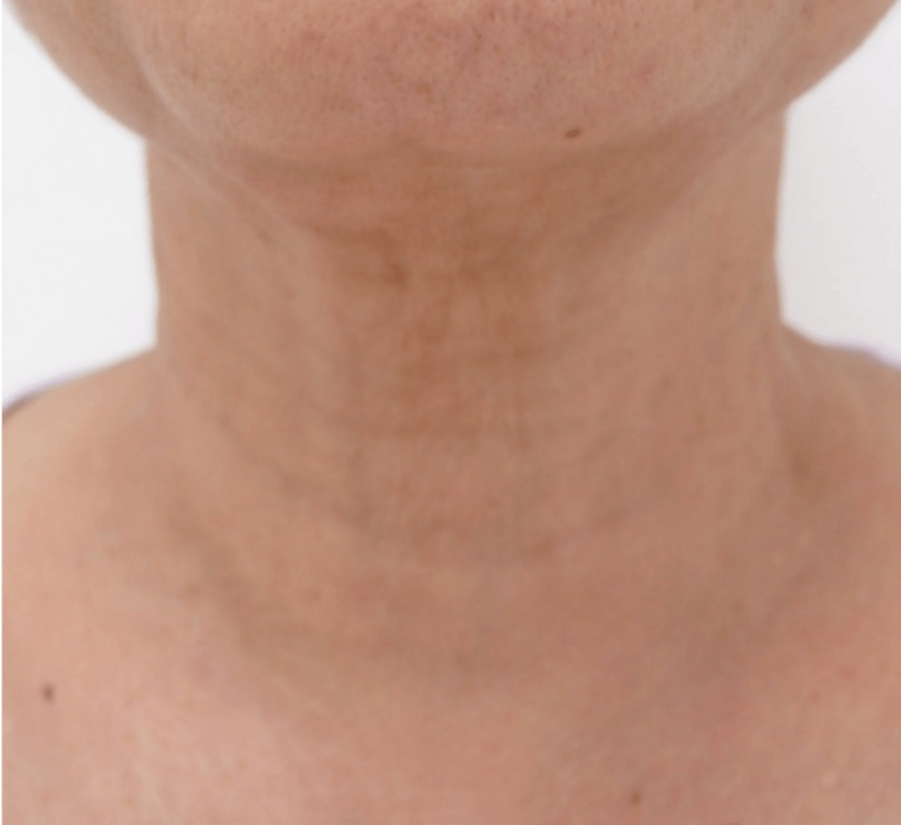
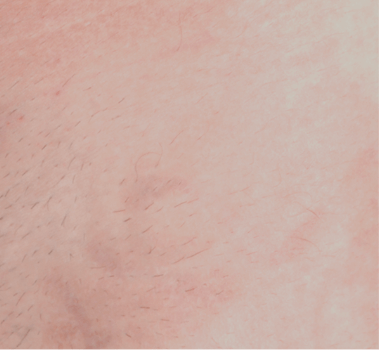
WRINKLES
Images are the property of VYDENCE® Medical
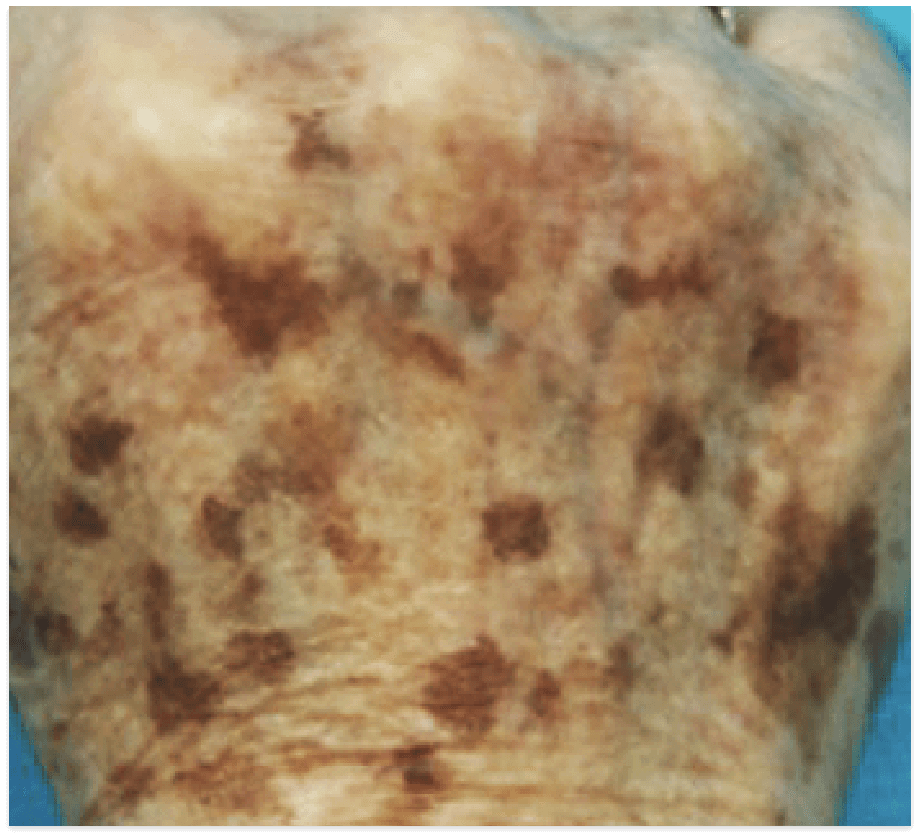
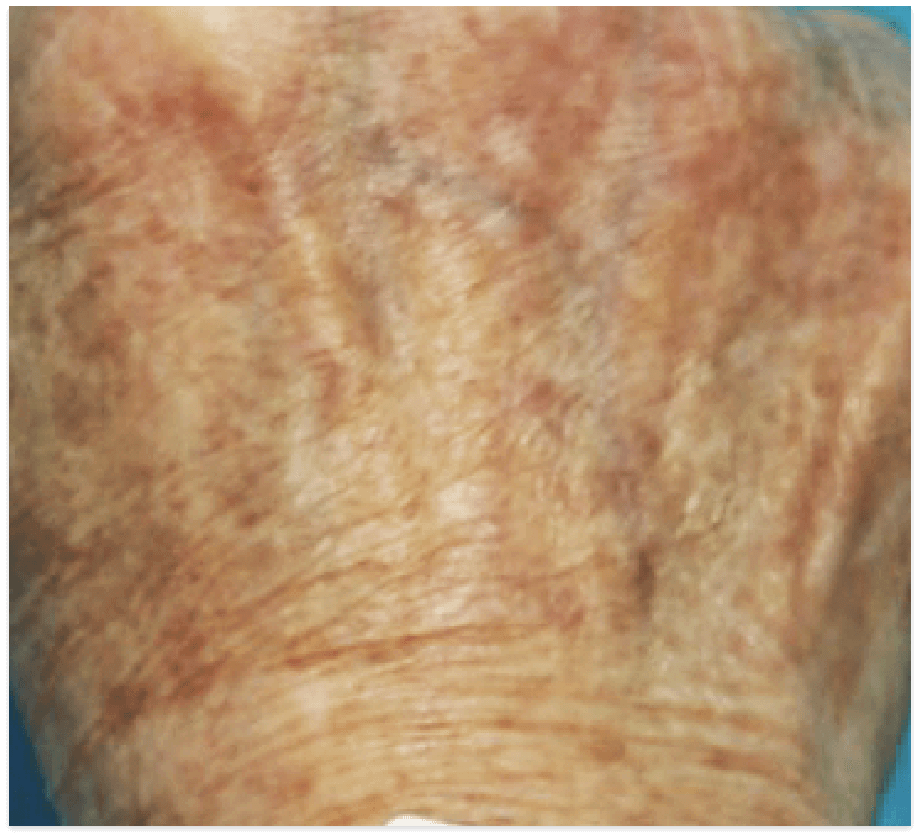
PIGMENTED LESIONS
Images are the property of VYDENCE® Medical
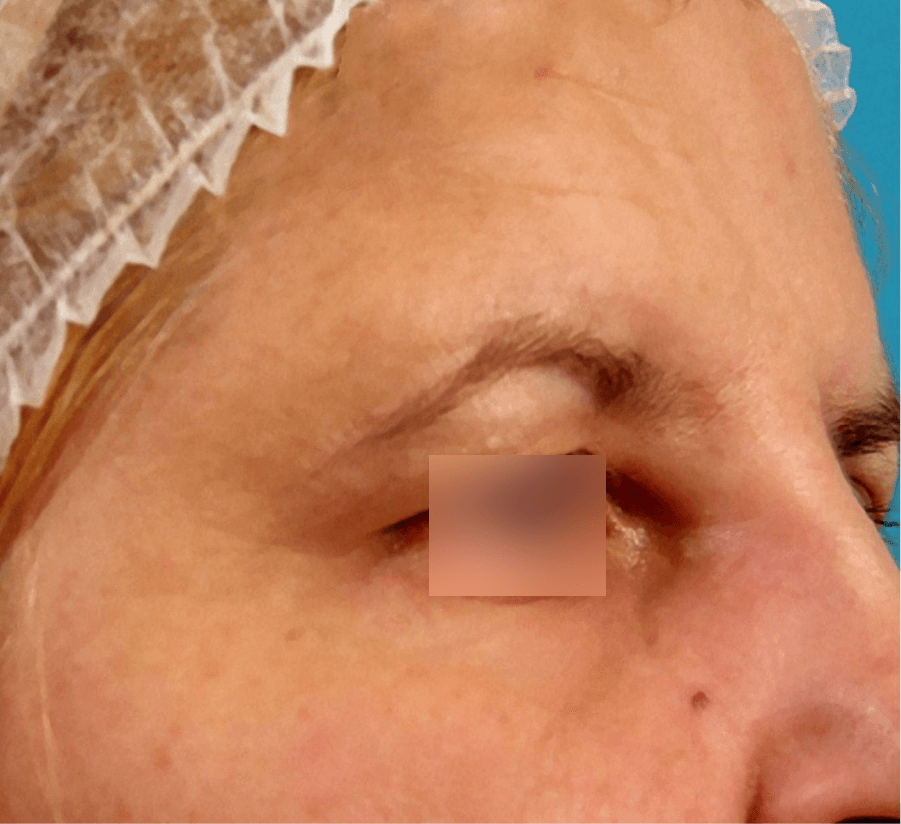
WRINKLES
Images are the property of VYDENCE® Medical
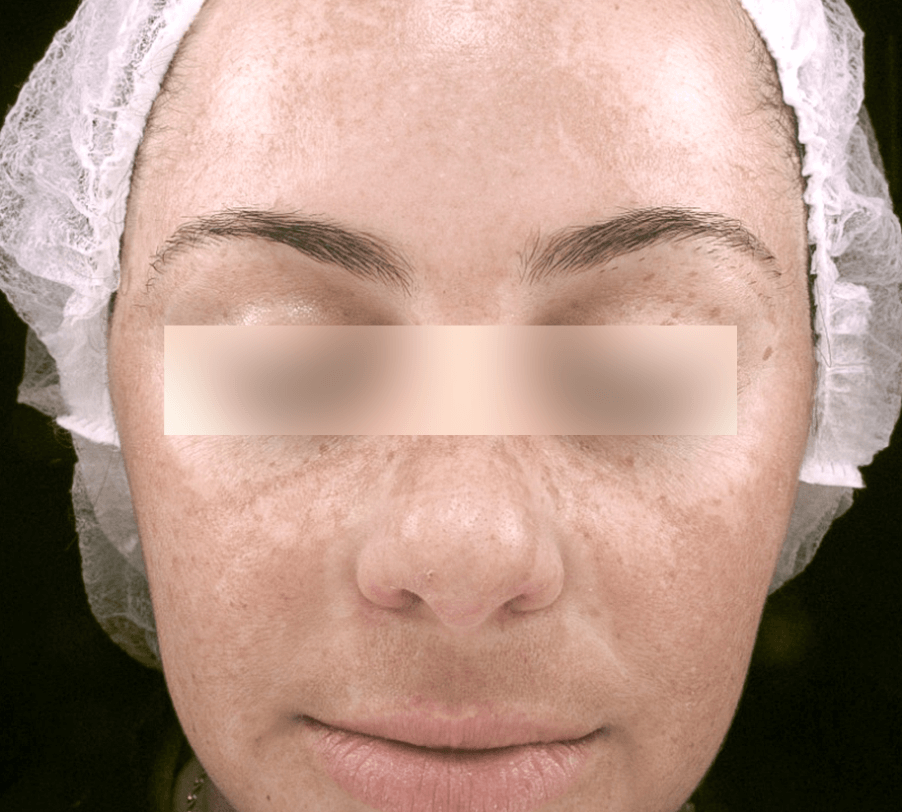
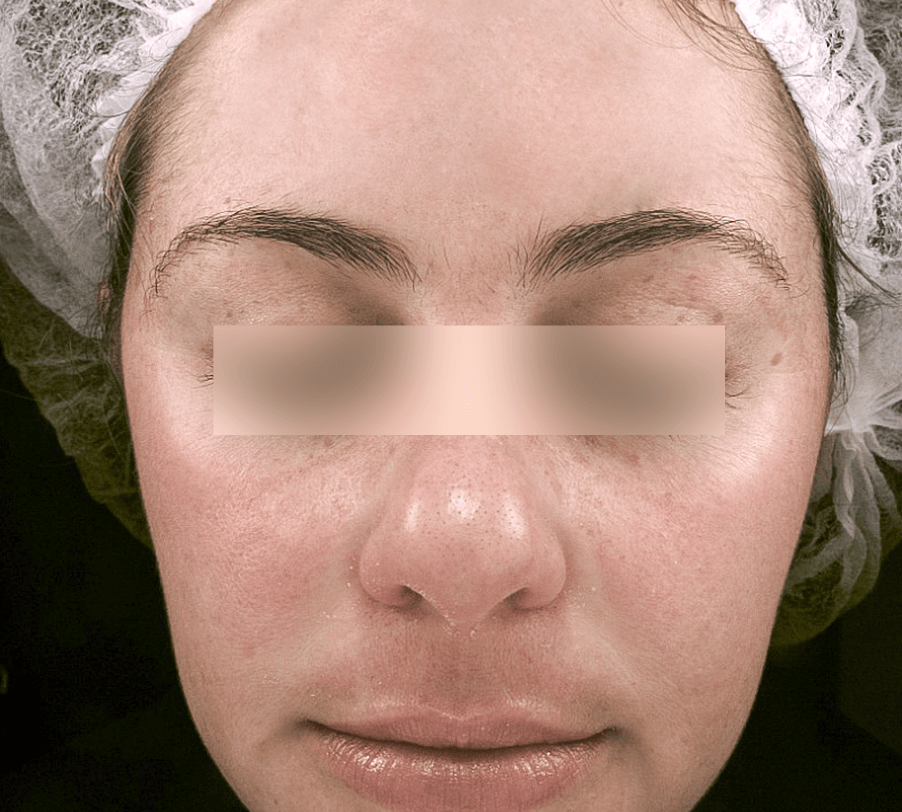
MELASMA
Images courtesy of Dr. Valéria Campos
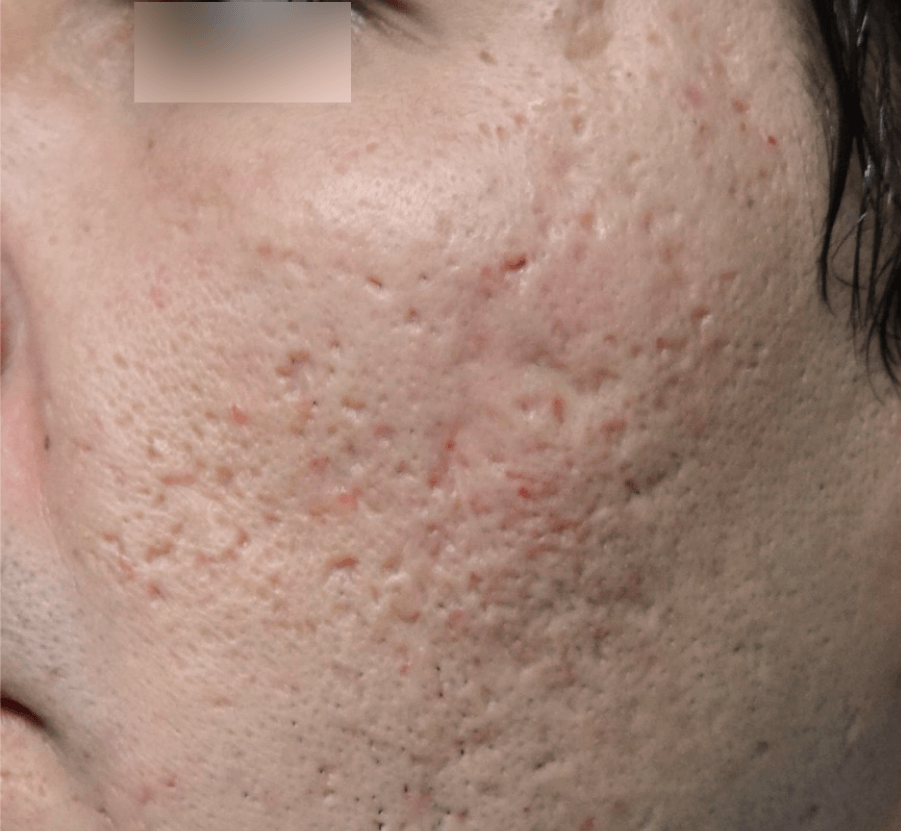
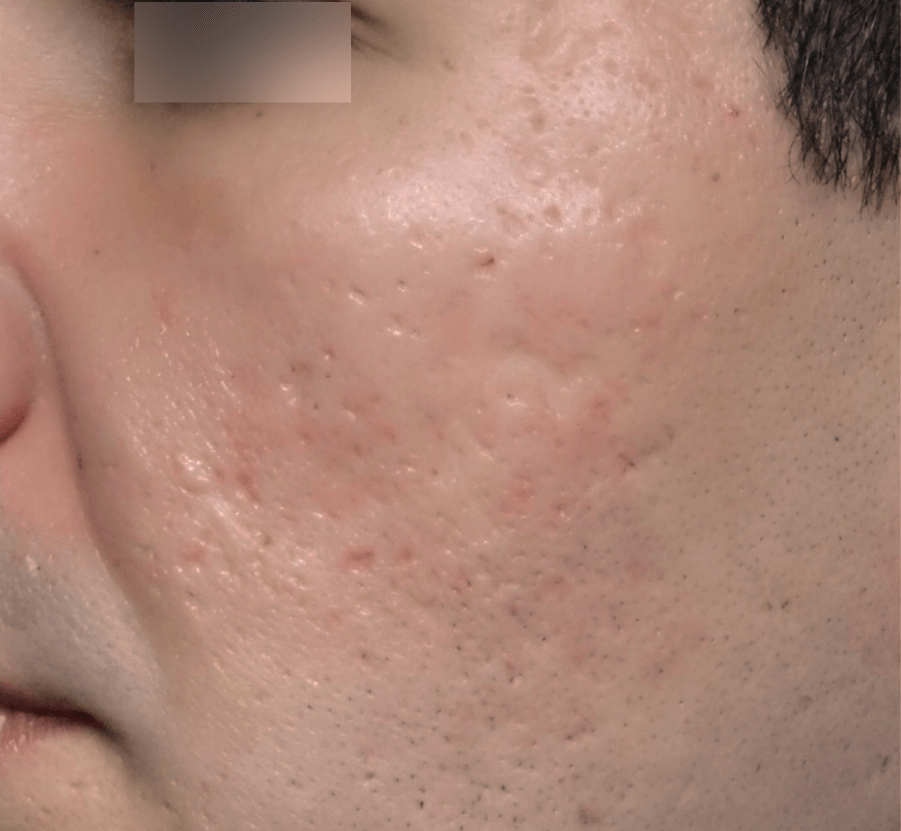
SCARS
Images courtesy of Dr. Marco Tulio de Oliveira
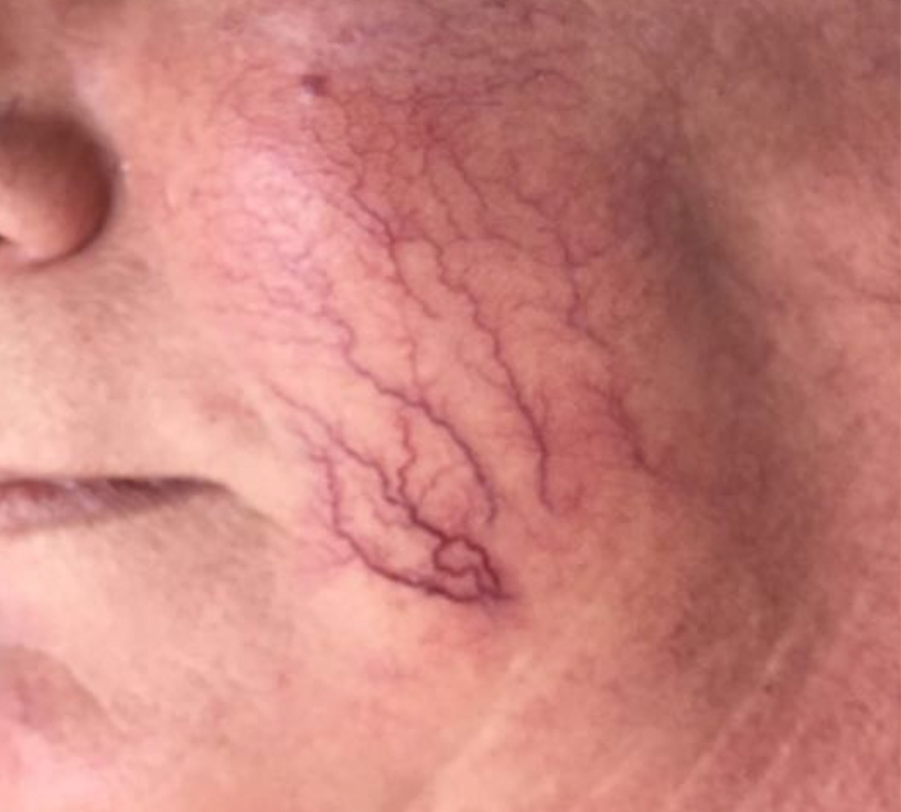
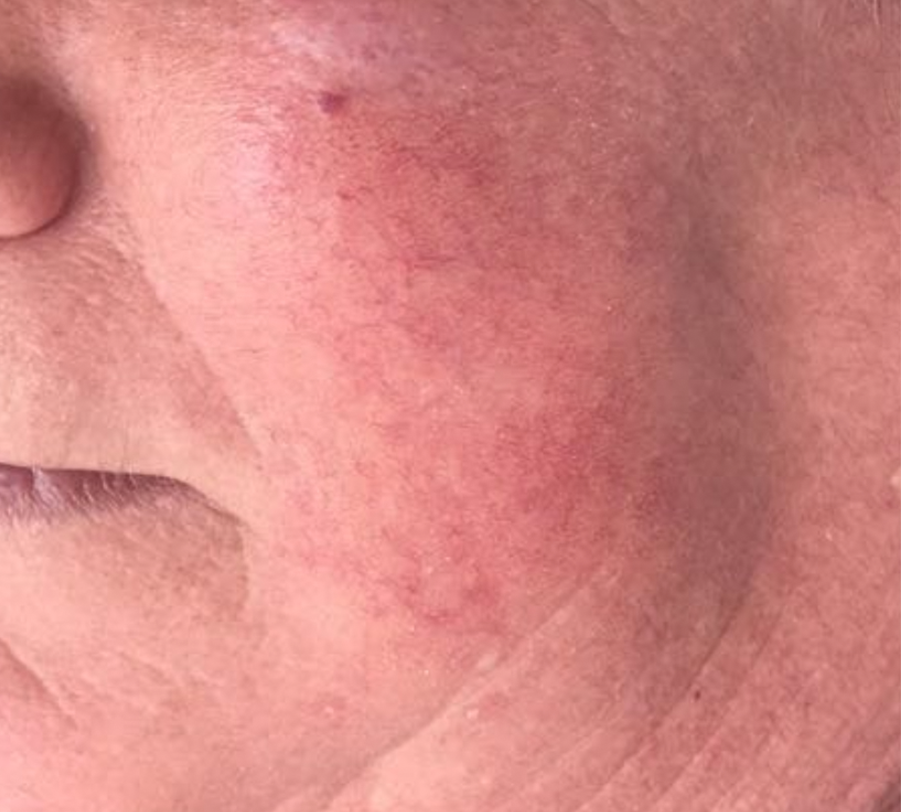
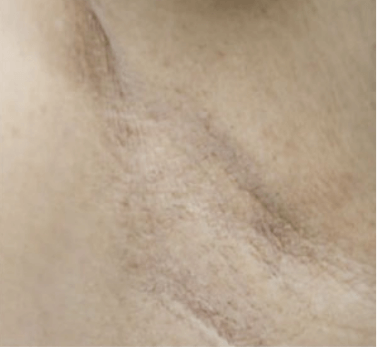
VASCULAR LESIONS
Images courtesy of Dr. Charles Esteves
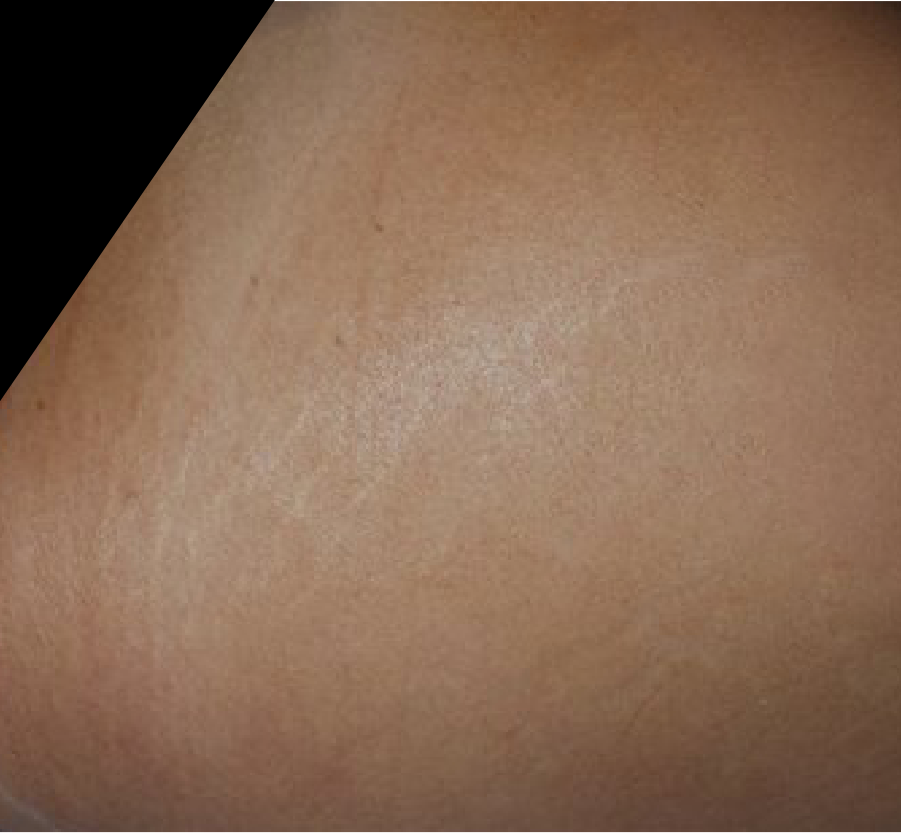
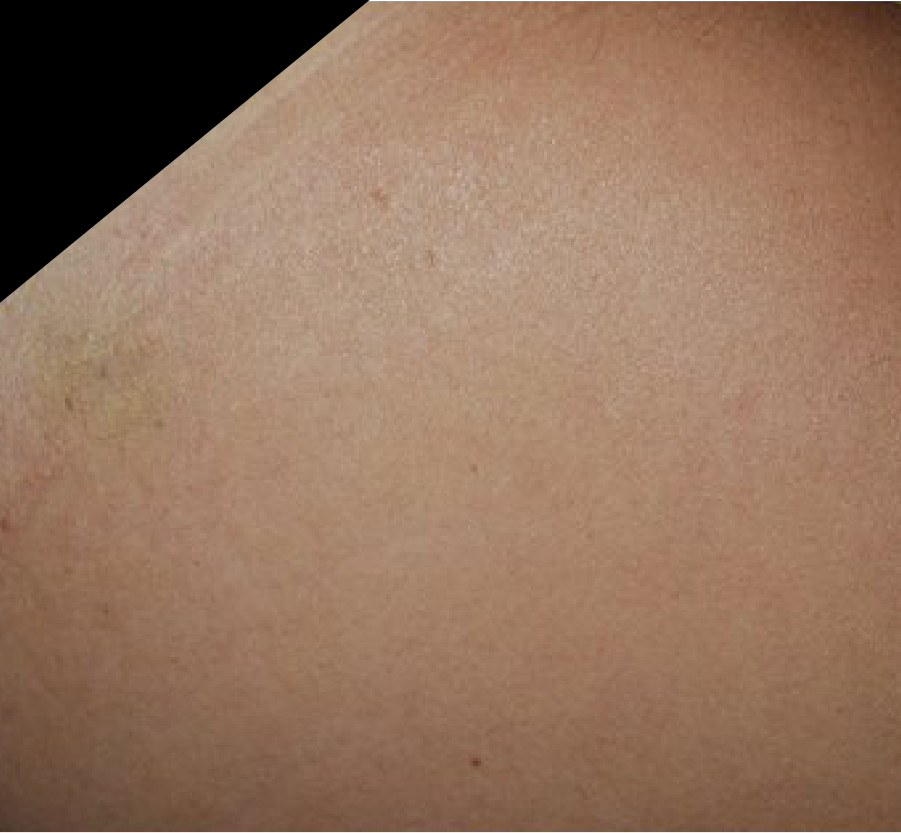
STRETCH MARKS
Images courtesy of Dr. Emerson Alves
SKIN TIGHTENING
Images courtesy of Dr. Valéria Campos
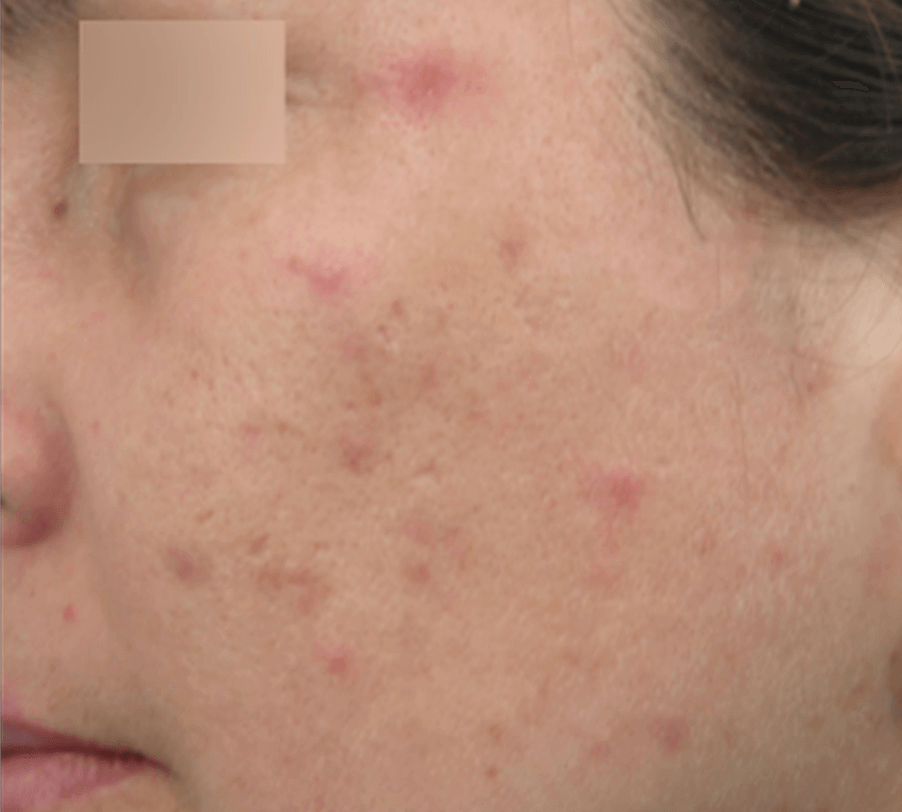
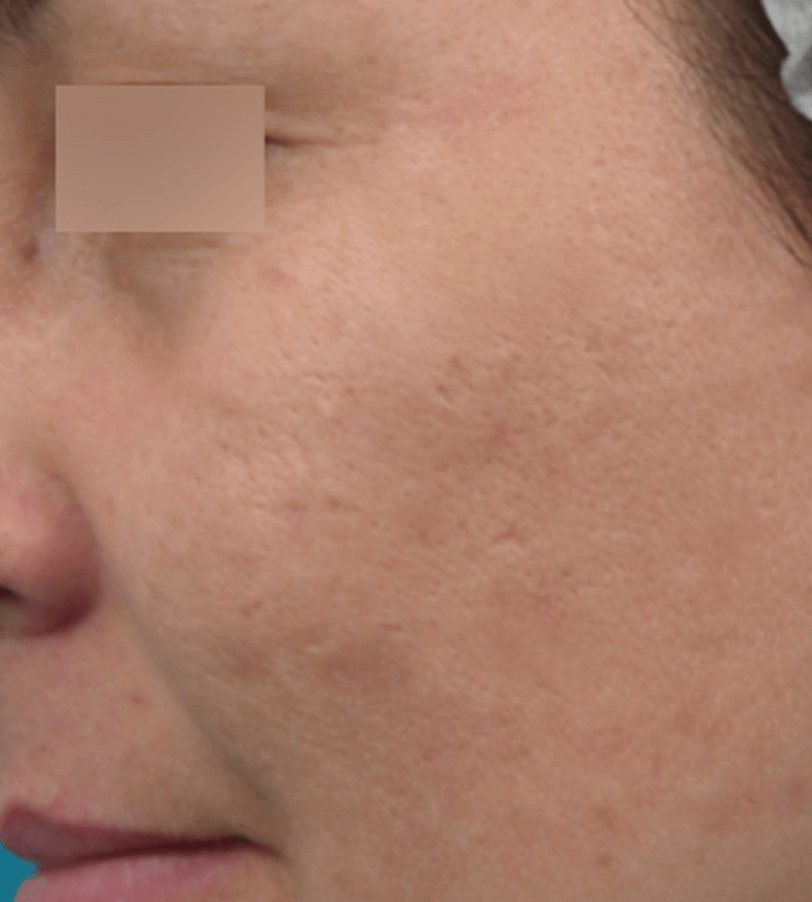
ACNE TREATMENT
Images are the property of VYDENCE® Medical
TATTOO REMOVAL
Images are the property of VYDENCE® Medical
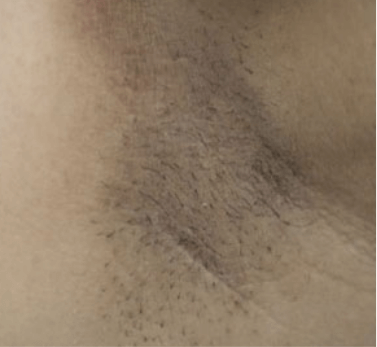
HAIR REMOVAL
Images are the property of VYDENCE® Medical
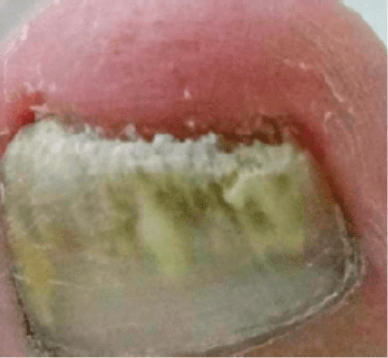
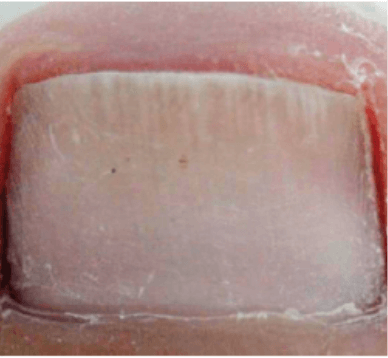
ONYCHOMYCOSIS
Images courtesy of Dr. Márcia Sansone

Learn more about the ETHEREA-MX®
DOWNLOAD THE CATALOG
SPEAK TO A SPECIALIST

These products and/or its stated treatments indications described in here might not be available in all territories/countries. Please, contact VYDENCE® for any inquiries regarding this matter. VYDENCE® reserves the right not to sell this product in territories/countries where it has no authorized distribution and/or marketing authorization by the relevant competent authorities.